HIV: Closer to the Cure
Researchers at Temple University make another HIV breakthrough using gene-editing technology, bringing us closer to a cure.

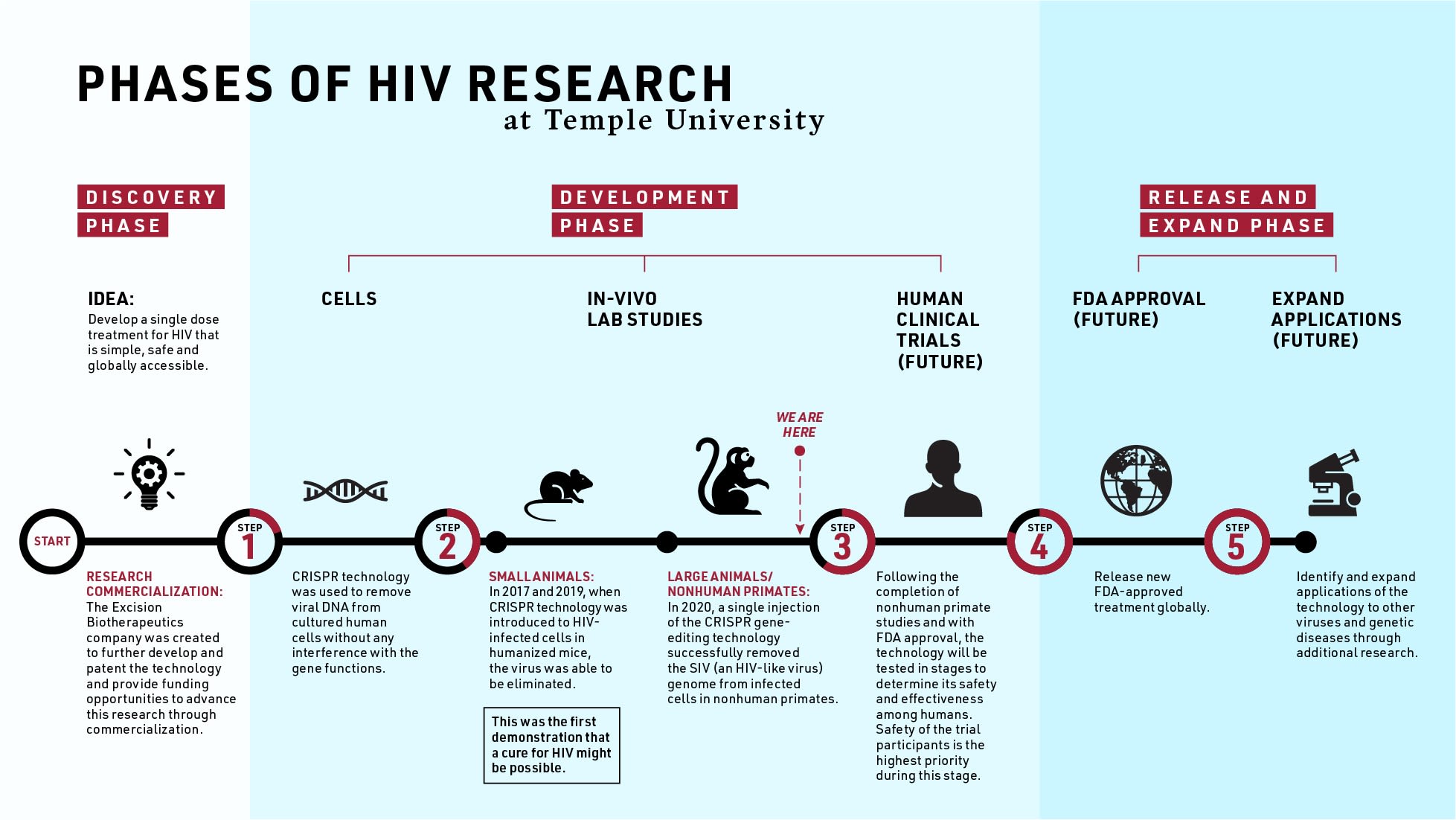
Researchers at Temple University have successfully removed viral DNA from primates, a crucial step that might lead to a cure for HIV.
A team led by Kamel Khalili, Laura H. Carnell Professor and Chair, Department of Neuroscience and Tricia Burdo, associate professor of neuroscience at the Lewis Katz School of Medicine, has used CRISPR genome editing to eliminate simian immunodeficiency virus (SIV), the primate equivalent of HIV, from a large animal model—the first experiment of its kind. The report was published online Nov. 27 in the journal Nature Communications.

Kamel Khalili, Laura H. Carnell Professor and Chair, Department of Neuroscience
Kamel Khalili, Laura H. Carnell Professor and Chair, Department of Neuroscience

Tricia Burdo, associate professor of neuroscience and associate chair of education, Department of Neuroscience
Tricia Burdo, associate professor of neuroscience and associate chair of education, Department of Neuroscience
HIV, which affects approximately 36 million people globally, attacks the body’s cells, replicating itself and integrating into the host’s genome, becoming part of the fabric of their DNA.
Scientists worldwide are exploring two different means of finding a cure. “One way is to find the infected cells and eliminate them completely. The second one is to find the infected cells and eliminate the integrated viral genome from the host’s DNA,” Khalili said.
Timothy Ray Brown, the first person ever cured of HIV and commonly referred to as the “Berlin Patient,” was treated using the first path: He received a bone marrow transplant that eliminated infected cells. But the therapy is difficult and a third of patients could die from undergoing a transplant.
Video production by: 20/20 Visual Media
The Temple team is following the second path—targeting infected cells and removing viral DNA—with a goal of developing a more simple treatment designed specifically for global accessibility. “CRISPR technology gives us the ability to excise viral DNA from the host’s genome without any interference with the host’s gene functions,” he said.
“What happens is that our technology cuts out the viral DNA and removes it,” Burdo said. “Your body will then end join the DNA back together and repair it.”
Antiretroviral therapy (ART) is currently the most common treatment for those infected with HIV. It suppresses the virus, but doesn’t eliminate it from a person’s DNA. “That’s a huge problem in the field, because that DNA then can result in proteins and more replication, and it can hide inside your body. We’re essentially cutting that part [of the DNA] out,” she said.
What is CRISPR?
CRISPR-Cas9 gene editing, more commonly known as CRISPR, allows scientists to precisely cut and alter strands of DNA. Emmanuelle Charpentier and Jennifer Doudna were awarded the 2020 Nobel Prize in Chemistry for developing the method, and Doudna collaborated with the Temple team on this study’s gene-editing system.
“It’s a fascinating technology if you think about [its] utility,” said Kamel Khalili, Laura H. Carnell Professor and Chair, Department of Neuroscience at the Lewis Katz School of Medicine.
He and his team are using it to target the viral genome that causes HIV-associated disorders. But researchers can apply the technique to other diseases as well, including cancer. “Of course you have to customize your method, you have to modify your CRISPR technology for that particular disease that you’re aiming [at],” he said.






Kamel Khalili and Tricia Burdo
Kamel Khalili and Tricia Burdo

Hong Liu and Kamel Khalili
Hong Liu and Kamel Khalili

Kamel Khalili, Chen Chen and Tricia Burdo
Kamel Khalili, Chen Chen and Tricia Burdo

Tricia Burdo and Kamel Khalili
Tricia Burdo and Kamel Khalili
Burdo specializes in the nonhuman primate model of HIV, while Khalili’s expertise is in gene editing. “We infected animals with SIV, which is the equivalent of the human virus,” she said. “And then we give them an in-vivo injection of CRISPR-Cas9 gene editing to edit out the proviral DNA.”
She and her colleagues have worked hard to ensure the technique they’re using is safe. “Obviously with any gene therapy, there are going to be risks. But it’s a calculated risk,” Burdo said. “We are doing extensive off-target analysis [looking for unintended genetic modifications that might be caused by using CRISPR] to ensure that this is going to be the safest product.”
They aren’t altering human genes; they’re cutting viral DNA, making sure it’s four or five mismatches from a human gene, so they avoid targeting healthy genes.
The neuroHIV field is small (the nonhuman primate one is even smaller) and no one has ever done an experiment like this before.
“The CRISPR technology that Dr. Khalili and his group have used started first in cells, moved on to animal models, and is now approaching the opportunity to perform clinical trials in patients,” said John Daly, interim dean of the Katz School of Medicine. “This is extraordinarily important to determine whether or not this new discovery will actually work in humans.”
If a person with HIV stops antiretroviral therapy, the virus rebounds, putting them at risk of developing AIDS and eventually, dying. “It’s like a Band-Aid strategy,” Khalili said. “We can control viral replication, but we cannot eliminate it [with ART].”
There are other problems with ART as well. “I think what is not really understood is that people with HIV that are on antiretroviral therapies for a long period of time have a lot of different comorbidities,” Burdo said. “They can have cardiovascular diseases, they can have brain abnormalities.”

Tricia Burdo
Tricia Burdo
A treatment based on the team’s research would work differently: a single infusion that people could get at a clinic or receive bedside.
And it’s important for it to be accessible because it could change so many lives. “I think in the U.S. and in Sub-Saharan Africa and Asia, there is a huge stigma still for HIV, and for people with HIV,” Burdo said. “Our technology will hopefully give the ability for these people to be HIV free.“
“It really sounded a few years ago like science fiction. Like, ‘Gene therapy for HIV? What are you talking about?’ But with CRISPR technology,” said Rafal Kaminski, assistant professor, neuroscience, “it is actually a real thing.”

Rafal Kaminski, assistant professor, neuroscience
Rafal Kaminski, assistant professor, neuroscience
Scientists have been working toward a cure for HIV for four decades; bringing one closer within reach is thrilling. “It’s just amazing,” he said. “It’s like a dream of every researcher that what you do actually matters, actually works.”
That dream is shared by Khalili’s entire group, a multinational, interdisciplinary team that’s also close-knit. “The level we’re [working at] right now,” Kaminski said, “you need a big team of committed, dedicated people and experts. And it’s great to be part of that team.”









Shuren Liao, researcher
Shuren Liao, researcher

Jake Robinson, researcher
Jake Robinson, researcher

Rahsan Sariye, researcher
Rahsan Sariye, researcher

Jennifer Gordon, researcher
Jennifer Gordon, researcher

Martina Donadoni, researcher
Martina Donadoni, researcher

Ilker K. Sariyer, researcher
Ilker K. Sariyer, researcher

Hong Liu, researcher
Hong Liu, researcher

Mandy D. Smith, researcher
Mandy D. Smith, researcher
“I’m very proud and motivated to do this research. I’m really happy I can help people.”

“If the results thus far are borne out, it will be a major step forward for individuals who no longer may have to take medication every day of their lives to suppress the virus, but indeed may finally be able to see the virus eliminated from them.”
“I consider Dr. Khalili like a rockstar in the field of gene editing. So it was an honor and a pleasure to join his lab.”

“I believe strongly in research collaboration across diverse groups: across our colleges, but also across academic medical centers, not only here in the U.S. but across the world,” Daly said. “This collaboration and the diversity of the research groups is critical to the discovery and then utilization of new advances in medicine.”
Khalili, Burdo and their team are currently working on the manuscript for a larger nonhuman primate study. After that, they’ll start laying the groundwork for clinical trials.
Their ultimate goal is finding a cure. “I would like to see that we can cure HIV right over here on North Broad Street,” Khalili said.

Jake Robinson, Shuren Liao, Rahsan Sariyer, Ilker K. Sariyer, Tricia Burdo, Kamel Khalili, Pietro Mancuso, Chen Chen, Rafal Kaminski, Mandy D. Smith and Martina Donadoni—all Temple researchers who contributed to the study.
Jake Robinson, Shuren Liao, Rahsan Sariyer, Ilker K. Sariyer, Tricia Burdo, Kamel Khalili, Pietro Mancuso, Chen Chen, Rafal Kaminski, Mandy D. Smith and Martina Donadoni—all Temple researchers who contributed to the study.
Other researchers contributing to the study include: Andrew MacLean, associate professor at the Tulane National Primate Research Center and the Department of Microbiology and Immunology at Tulane University School of Medicine; Binhua Ling, associate professor at the Southwest National Primate Research Center, Texas Biomedical Research Institute; Pietro Mancuso, Chen Chen, Jennifer Gordon, Shuren Liao, Jake Robinson, Mandy Smith, Hong Liu, Ilker K. Sariyer, Rahsan Sariyer and Martina Donadoni, Department of Neuroscience, Center for Neurovirology, Lewis Katz School of Medicine at Temple University; the late Tiffany Peterson, Jaclyn Williams and Summer Siddiqui, Division of Comparative Pathology at the Tulane National Primate Research Center; and Bruce Bunnell, Division of Comparative Pathology at the Tulane National Primate Research Center, the Tulane Brain Institute, the Department of Pharmacology at Tulane University School of Medicine, and the Department of Microbiology, Immunology and Genetics at the University of North Texas Health Science Center, Fort Worth.
Read more about research at Temple University.

